Looking to expand your partner network with the latest in the field of CRISPR? Consider joining Inpart's global network for free.
Stay up-to-date with the latest news related to CRISPR. Subscribe to our newsletter.
What is CRISPR?
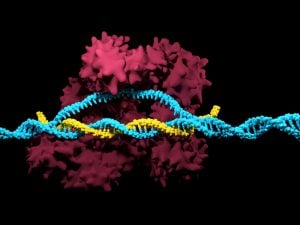
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a revolutionary gene-editing technology that allows scientists to precisely edit DNA sequences in living organisms. It uses an enzyme called Cas9, guided by RNA, to cut specific genes and replace them with new ones.
What is CRISPR used for?
CRISPR has a wide range of potential applications, including developing new treatments for genetic diseases, improving crop yields, creating disease-resistant livestock, and advancing research in biotechnology and synthetic biology.
Got a news story for us related to CRISPR? Send it to us here.