Ernest Loumaye began as a doctor at a university hospital and is now CEO of ObsEva. We chatted about how biotech supports women’s health.
ObsEva, one of the top biotechs in Geneva that made this year’s first IPO for a huge €90M, is not Ernest Loumaye’s first company. Though he began as the gynecologist in charge of the reproductive unit at a university hospital in Brussels, he co-founded PregLem in 2006 to develop and commercialize reproductive medicine for women. In 2010, he saw through its acquisition by Gedeon Richter for CHF 445M (€337M), right as the company was preparing for an IPO.
Two years later, Loumaye moved on to found ObsEva with the same focus on women’s health. Since its first funding arrived in the summer of 2013, the company has grown to boast two Phase III candidates, one for uterine fibroids and another for IVF, as well as others for endometriosis and preterm labor.
I caught up with him to hear more about how the industry treats women’s health and what inspired him to go into the area.
What is it about gynecology and obstetrics that specifically interests you, and why did you go into biotech from academic medicine?
It’s an area where you do a range of medicine — you do a bit of surgery, endocrinology, and psychology. It’s really a discipline where you have broad practice and impact. Also, (I think many people have this reason,) you’re working with young people who are not necessarily very sick and the end result is a baby.
It’s new life! It’s very rewarding from an emotional standpoint, and a very optimistic practice compared to other disciplines, though they’re all obviously very worthwhile. On the other hand, gynecology is also very demanding, because you’re on call day and night — you can’t really pick your office hours. That makes it tough if you want to have a family…

I initially did not aim to go into the industry, because I’ve always been interested in taking care of patients and doing research. But I realized that industry is really providing a huge resource to discover and develop new drugs. I find it extremely attractive that I can stay in my area of interest as a gynecologist and deliver something more broadly applicable and concrete than making observations that perhaps one day could be useful.
I’m also a strong believer that having a clinical background in your target therapeutic area is a big advantage, not only because you have to know your stuff but also because it helps avoid misunderstandings and improves your credibility with physicians and regulators. I don’t believe developers in the industry can just pick any therapeutic area and make a drug for it.
But back to the question — as we go to market with our lead candidate, there are already hundreds of women benefiting from it, and I find that extremely satisfying. To summarize, in a word, it’s the innovation that could help millions of people that attracted me to the industry.
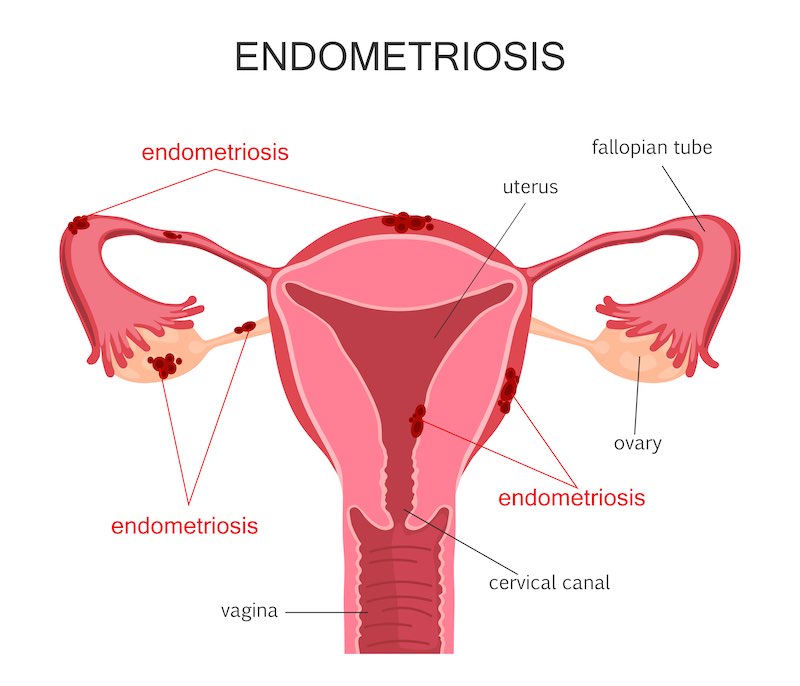
For the treatments for heavy menstrual bleeding, how would you respond to critics who might argue that it’s just a quality of life issue?
Treating fibroids is much more than just a question of quality of life. This is already recognized by authorities as such…At PregLem, we obtained reimbursement for our drug treating fibroids in most European countries.
Indeed, 40% of women with fibroids and heavy menstrual bleeding already have some level of anemia — 12% have severe anemia. The bleeding is just the symptom of the more serious issues of pain and anemia. In addition, there is, of course, the cost to productivity at work.
Without medical treatment, surgery is the only option and fibroids are still indeed the leading cause of hysterectomy. In the US, for example, there are still 500 thousand of these surgeries per year, and 200 thousand come from fibroids. Hysterectomies of course have immediate risk like any major surgery, and they may have long-term complications, like urinary incontinence; but women can also be reluctant to undergo them because it’s perceived as losing some physical integrity. Nevertheless, I don’t think that surgery will disappear, but we need to avoid it as much as possible.
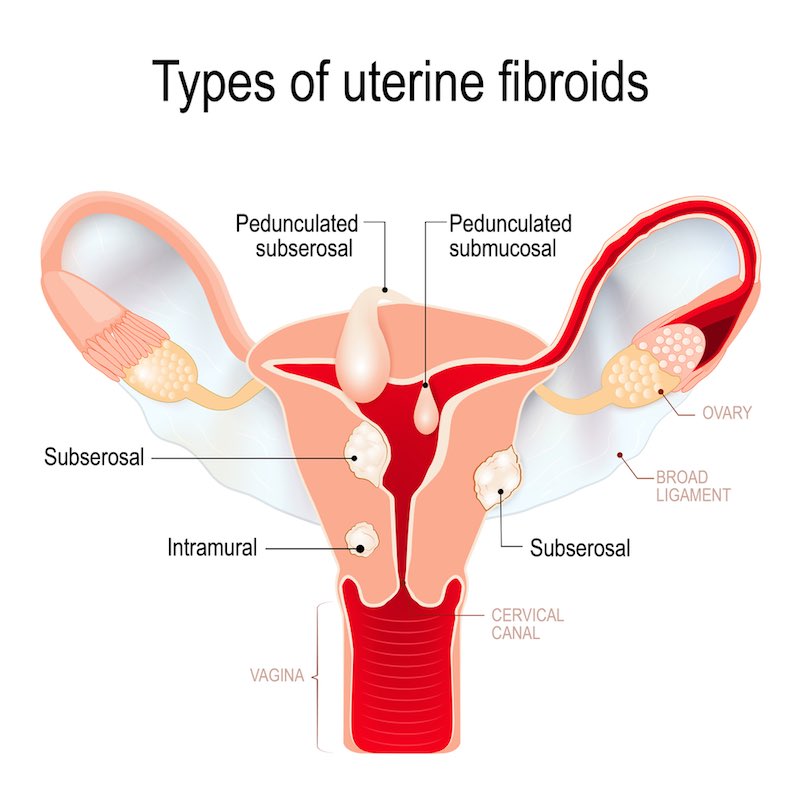
Why is it your sense that women’s health is an overlooked area in biotech?
It’s true that people pay more attention to oncology or to another disease such as diabetes, and women’s health has been regarded by the industry as an area with small market potential and not so much innovation. We’re still living with the generation of drugs discovered in the 70s and 80s, which now have cheap generics. Even though the number of subjects is large, the competing drugs are very cheap.
This has been changing though… We now see companies like Pfizer, Abbvie and Allergan paying more attention to women’s health addressing conditions like endometriosis and fibroids. Merck has been doing large business in fertility treatments.
For the fertility, as women gain education and earning power, they delay having children and end up running against time to have a child. When you’re a 35- to 45-year old woman, your chance of infertility is much higher than when you’re in your 20s, so there is an increasing need all over the world.
In this context, we are seeing a boom in demand for treatment, particularly in China as the country recently change the one-child policy to a two-child policy. The Chinese market is much larger than that of the US: 20 million people are infertile, and there are an estimate of 800 thousand IVF cases per year — four times the number in the US.
Images via Magic mine, Fancy Tapis, HelloRF Zcool, Designua / shutterstock.com