Money doesn’t guarantee success, even if it’s heavy investment from the likes of the Bill & Melinda Gates Foundation. Ingmar Hoerr explains what it means for mRNA therapeutics.
In a blow to the field of mRNA, the German billion-euro biotech CureVac has reported the Phase II failure of its lead candidate at JP Morgan. Aimed at prostate cancer, CV9104 did not meet its primary endpoint of improving overall survival, though it achieved a secondary safety endpoint.
That counts for something, given the toxicity issues CEO Ingmar Hoerr discussed at Refresh last year; unfortunately, this new result “is not what we hoped to get,” he told us, “but on the other hand, it was clear to us that it was a high bar.”
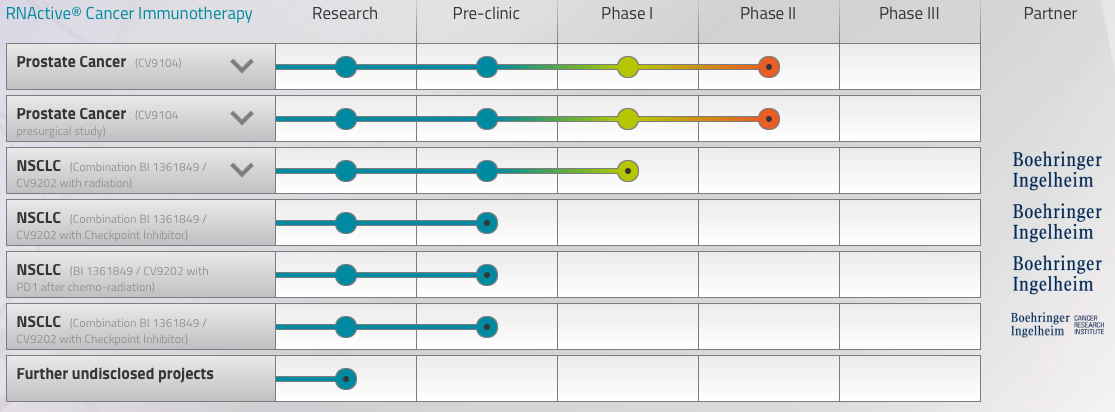
This result forecast that mRNA therapies may never be able to stand alone, but despite the bad news, Hoerr remains positive. “What we’ve learned here is that mRNA is not enough on its own — you have to break tolerance and you have to make it more immunogenic.” Hoerr, as well as his rivals at Moderna and BioNTech, sees a solution to this problem in checkpoint inhibitors.
“In 2012, we moved our therapies into Phase IIb based on interesting immunodata in Phase IIa studies, but we didn’t include checkpoint inhibitors because they weren’t available yet,” he says. “They are now, and we currently have a combined therapy that appears safe in animal models. We’re already planning with our partner, Boehringer Ingelheim, to start clinical trials of mRNA in combination with checkpoint inhibitors.”
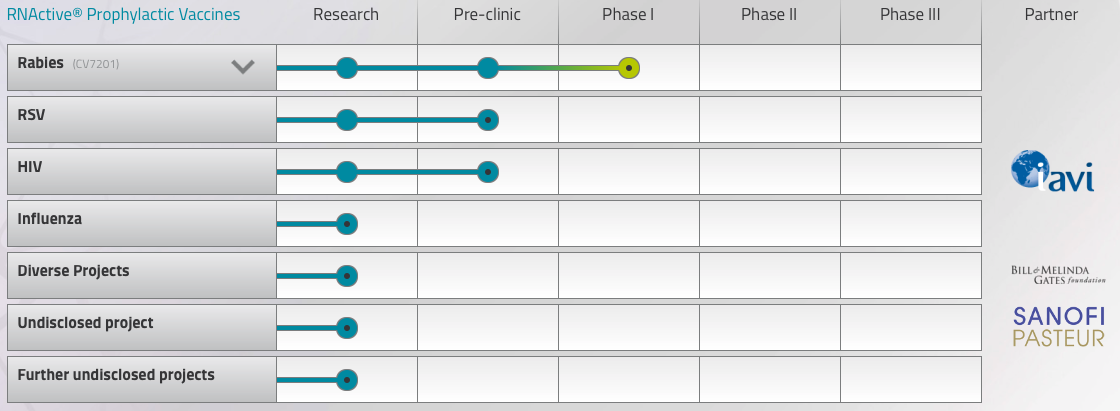
Sitting pretty atop a cash pile of €355M, CureVac has the financial muscle to carry out these plans. More importantly, the company also has an additional trick up its sleeve, a rabies vaccine. CV7201 has returned positive interim results in first-in-human Phase I trials and appears tolerable and safe.
Beyond the progress towards a faster and cheaper prophylactic, Hoerr says that CV7201’s results represent validation of the company’s proof of concept: “In the rabies vaccine, we saw that we have an active substance — the first-ever proof of concept — which is the same formulation as in the cancer study. Since we were able to prime an immune response in naïve healthy patients, we can say, mRNA works!”
With a prototype is in hand, Hoerr says the company can build upon it to make “the best product ever, one that’s very safe and has good expression of mRNA.” And he’s not so worried about the competition, since “the field is still investigating: everyone has to validate a concept before we talk about product design.”
Despite the omen for the whole field that mRNA isn’t strong enough to make it as an independent therapy, CureVac’s main competitor, Moderna, stands to make a short-term gain in the race, if the bubble doesn’t burst out from under its feet. As detailed in what might be The Press Release of the Year, this golden child poses a threat with an arsenal of early-stage mRNA therapies comprised of candidates for influenza and Zika to Phase I — its cancer pipeline is still in the preclinical stage — and a whopping a €1.75B stash.
A second competitor is BioNTech, another one of the select few European biotechs chosen to present at JP Morgan this year. Its Individualized Vaccines Against Cancer (IVAC) platform is also making its way through Phase I, now with the help of Genentech.
Still, no one has come as far as we have. The most important thing is that you engineer your RNA before you get to product development, and that’s the stage where we are right now.” Ingmar Hoerr, CEO of CureVac
Images from VERSUSstudio / shutterstock.com and CureVac





