Regeneron and Sanofi’s drug, dupilumab, has met primary and secondary endpoints for the treatment of eosinophilic esophagitis.
The biotech-biopharma giants have announced that their candidate, Dupilumab, demonstrated good efficacy during a Phase II trial. The drug is designed for use against eosinophilic esophagitis, a nasty inflammatory disease of the esophagus that makes it difficult to swallow. In comparison with a placebo, the drug significantly improved the ability of patients to swallow. The companies hope to continue moving dupilumab towards the clinic to provide an effective treatment targeted at the disease.
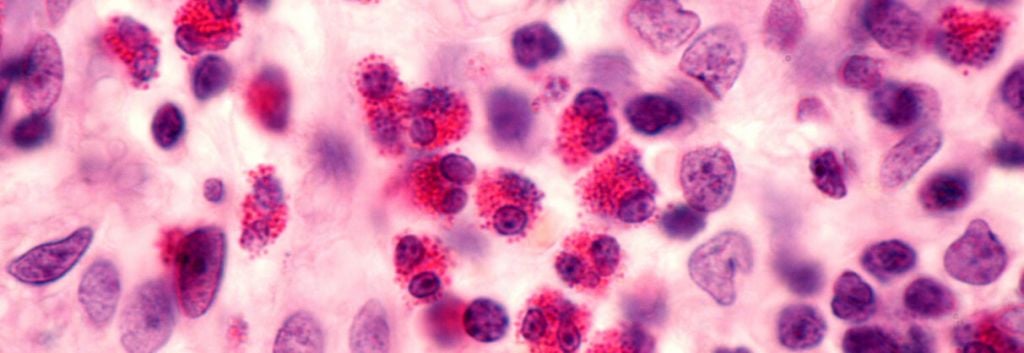
Eosinophilic esophagitis is characterised by a build up of eosinophils in the esophagus. The symptoms vary with age, for example, young children can refuse food and not grow properly while teenagers and adults struggle to swallow dry or dense solid foods. Ultimately, food can become stuck in the tube, which is known as food impaction, which is a medical emergency.
Dupilumab is an antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13). IL-4 stimulates naive helper T cells, IL-13 is involved in B cell maturation and differentiation, and both are implicated in type 2 allergic inflammation. The drug has been approved for moderate-to-severe atopic dermatitis in adults and has achieved positive Phase III results for the treatment of asthma and nasal polyps.
Roles of IL-4 and IL-13 in inflammation.
After 12 weeks of treatment, the drug met its primary endpoint, causing a significant 45% improvement in patient swallowing according to a patient-reported measure. Secondary endpoints, including eosinophil numbers and scarring of the esophagus, were also significantly reduced by 93% and 48%, respectively. No safety concerns were observed.
There is a lack of therapies for the effective treatment of eosinophilic esophagitis, with options limited to diet modification, corticosteroids and surgery. IL-4 and IL-13 have become popular targets for companies developing drugs for inflammatory conditions. For example, Leo Pharma bought tralokinumab for the treatment of atopic dermatitis and asthma in a deal worth $1B (€900M) – which is in blockbuster territory, assuming the drug rakes in more than this price in sales.
Dupilumab received orphan drug designation for the condition and the fact that Regeneron and Sanofi have already enjoyed success with the drug means it could well march quickly onto the market. Despite this, the power-couple have decided to split at the end of the year.
Images – Jose Luis Calvo / shutterstock.com; Oh et al. (2010).