The ultra-fast diagnostic instruments developer, GNA Biosolutions (GNA), started the FILODIAG (Filovirus Diagnostics) project for developing a high-speed Ebola detection system based on GNA’s novel Laser PCR technology.
There is an urgent need for fast and accurate diagnostic tests in the current and any future Ebola crisis. The rapid diagnosis of Ebola during early and late stage of infection is a decisive step for risk assessment and for guidance to physicians to take the necessary decisions to limit the spread of the infection, and to safely nurse the infected patients. While fast and easy-to-use tests usually rely on immuno-diagnostic approaches, they typically lack high sensitivity and specificity. The gold standard for accurate diagnostics is Real-Time PCR but this procedure requires special laboratory facilities and a long processing time of up to several hours. The aim of the FILODIAG project is to deliver a diagnostic system fast enough for point-of-need testing of incoming patients.
The core technology being used is based on GNA’s laser-heated nanoparticles (Laser PCR) that helps to overcome the time-limiting step of heating and cooling the reaction sample in conventional PCR reactions. GNA have revolutionized this standard procedure by inducing the necessary temperature cycles with laser-heated nanoparticles that can be heated and cooled more than a million times faster than in conventional PCR. GNA has already performed Ebola Laser PCR assays that detect 10 target copies of synthetic nucleotides, corresponding to the Ebola genome sequence, in less than 12 minutes.
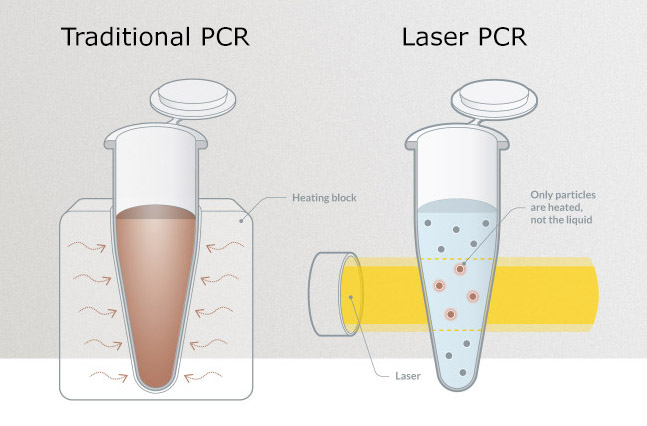
Project coordinator Dr. Lars Ullerich, a Managing Director of GNA, said: “We are working with our international partners to develop a highly sensitive and specific Laser PCR assay based on saliva, urine or blood for Ebola detection. Our proprietary Laser PCR with ten times faster cycles allows us to utilise the gold standard of PCR also in Ebola diagnostic. Together with a label free detection, the test results will be available in less than 15 minutes. Our system can already detect other highly dangerous pathogens within three minutes and a rapid, simple testing workflow will be crucial to deliver effective support in the management of Ebola outbreaks.”
The FILODIAG project is being funded with €2.3M by the Innovative Medicines Initiative 2 (IMI2). This initiative receives support from the European Union’s Horizon 2020 research and innovation programme. IMI2 has recently launched the programme Ebola+, in which eight funded projects have been announced, including FILODIAG, and two further projects with a diagnostic focus.
Ebola may not be in the front pages anymore, but the drama is far from over. With the worst propagation of ebola virus in History, not even the experts dare to predict when the end will be.