Oslo based Biopharma Bionor has a Phase I results for a HIV vaccine. The vaccine infusion rendered viral presence in 88% of patients undetectable after combination treatments with Celgene‘s enzyme inhibitor, Istodax and standard retroviral drugs.
![]() The positive results from the clinical trial REDUC Part B were published in the high impact journal The Lancet HIV.
The positive results from the clinical trial REDUC Part B were published in the high impact journal The Lancet HIV.
The Norwegian’s proprietary vaccine works as a therapy in conjunction with other drugs to ‘work towards a functional HIV cure’.
The vaccine (Vacc-4x) was tested in a Phase Ib/2a trial in combination with Celgene’s Istodax (romidepsin), well known cancer specific cytotoxic protein. Adults who were infected with HIV and already receiving cART (combination anti-retroviral therapy) for over a year were recruited for the trial at the Aarhus University hospital in Denmark.
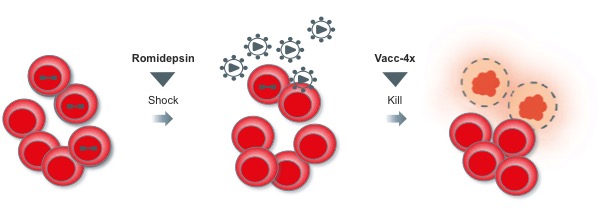
Vacc-4x is designed to induce CD4+ and CD8+ T-cell responses, to stimulate the immune system to remove HIV-infected cells. Romidepsin on the other hand is a natural product of the soil bacterium Chromobacterium violaceum, which works to block enzymes responsible in cell apoptosis (as a Histone deacetylase inhibitor).
The trial concludes that the REDUC trial was safe and worked in its ‘Shock and Kill Strategy’ for the virus. Romidepsin acted as a ‘shock’ for the virus to leave its latency state, and then the immune system was primed with the virus to attack and remove the viruses.
Any adverse effects were also already known to be associated with either romidepsin or the Vacc-4x.
The virus reservoir latency in the patient was reduced by around 40%, which was measured by how much HIV DNA was present and qVOA (quantitative viral outgrowth assay).
Secondly, viral load for HIV-1 RNA became undetectable (20 copies/ml) after a single infusion of the therapy in 65% of the patients, whilst in the remaining 35% the viral load that was detected was very low. And this was after just 1 go!
But more importantly, after each of the total 3 romidepsin infusions, just 2 patients (12%) had any viral load detected at all. However, as you may have noticed from the proportions, the trial size is clearly very limited in size and scaling up for future trials will give more accurate results on how effective the treatment is.

Other trials (of which there have been 7 in total) have been carried out in the UK, Germany, Norway, Spain and Italy, in over 260 patients overall. Bionor also claims Vacc-4x treatment is scalable, as manufacturing of the peptides is fairly straight forward.
Other biotechs with HIV vaccine related news include Abivax, which also claims to have the “the World’s Most Advanced Functional HIV Cures under development”.
Despite this, results also concluded that more needs still to be done to improve the virus clearance. But if managed, it could mean this could really make a difference to HIV patients.
Feature Image Credit: © 2011, Charlotte Raymond Photography for International AIDS Vaccine Initiative (IAVI)