Merck and Pfizer have been granted priority review by the FDA for the first treatment dealing with one of the most aggressive forms of skin cancer.
The FDA has accepted Merck and Pfizer‘s market application for avelumab in metastatic Merkel cell carcinoma (MCC) with priority review. Just a month ago, the EMA also accepted their application. If approved, this would become the first approved treatment for MCC, a rare neuroendocrine skin cancer with a survival rate lower than 20% over five years.
The results of avelumab in Phase II were impressive: 30% of MCC tumors resistant to previous treatments shrank or fully disappeared. The antibody drug targets PD-L1, a checkpoint inhibitor all big pharma seem to be after.
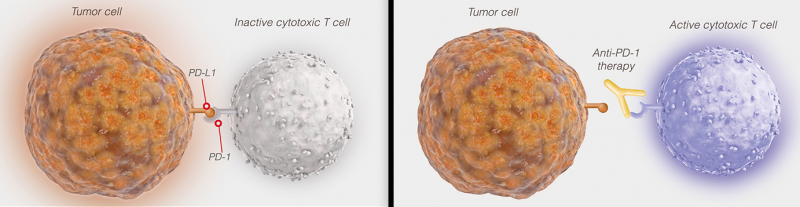
Avelumab could make a big difference for the 2,500 Europeans diagnosed with MCC each year. Also, it is estimated to bring Pfizer peak sales of €1.8B ($1.9B). For its part, Merck received €800M ($850M) as an upfront payment back in 2014 and could receive up to €1.9B ($2B) in milestones.
The drug is also performing well in clinical trials for non-small cell lung cancer (NSCLC), recurrent ovarian cancer and bladder cancer. However, Pfizer and Merck will have to face strong competition in these indications, since checkpoint inhibitors have proved to be one of the major success stories of immuno-oncology.
Roche‘s Tecentriq (atezolizumab), approved for bladder cancer in May and for metastatic non-small cell lung cancer in October, is the only PD-L1 drug currently on the market. If approved, Avelumab would become the fourth checkpoint inhibitor drug in the market, after Roche, BMS and MSD. BMS’s Opdivo (nivolumab), also targeting PD-L1, is hot on its heels with positive Phase III results for head and neck squamous cell carcinoma (HNSCC).
Competition, however, seems to be a good incentive for big pharma to make ever more effective drugs, which ultimately benefit the patients, so bring it on!
Featured image by Crevis/shutterstock.com; figure from Agilent