Sanofi and Regeneron (NY, US) have ruled their phase III clinical trial immunotherapy Sarilumab a huge success. Now awaiting US FDA and EMA approval, the results from the latest study were presented at the American College of Rheumatology (ACR) annual meeting in San Francisco.
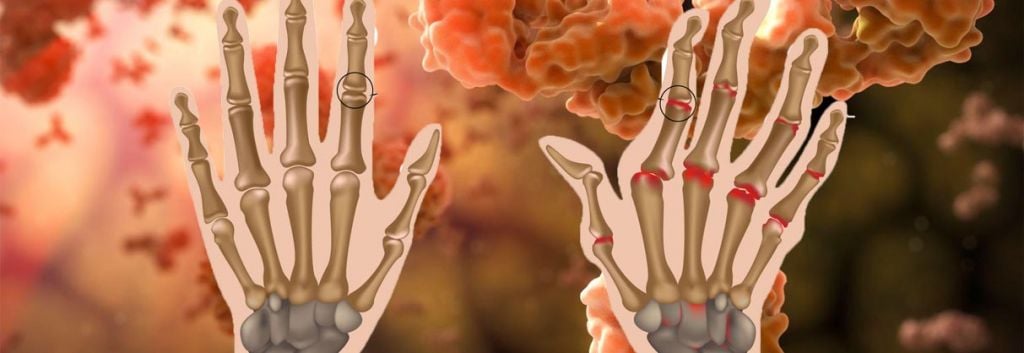
 Should Sarilumab be approved, this immunotherapy will be used to treat sufferers of mild to severe Rheumatoid Arthritis – a debilitating and painful chronic disease. These phase III trial results are therefore great news, having succeeded in both co-primary endpoints: improvement in symptoms of Rheumatoid and improvement in physical function of patient joints.
Should Sarilumab be approved, this immunotherapy will be used to treat sufferers of mild to severe Rheumatoid Arthritis – a debilitating and painful chronic disease. These phase III trial results are therefore great news, having succeeded in both co-primary endpoints: improvement in symptoms of Rheumatoid and improvement in physical function of patient joints.
There is a strong competition for antibody research into chronic auto-immune diseases such as Rheumatoid. A few Biotech giants are extra anxious to make new breakthroughs in the field, seeing as some of their Rheumatoid blockbuster patents will soon expire (i.e. AbbVie and Johnson&Johnson), exposing their sales territory to the merciless Biosimilar…
Besides, the complexity of this auto-immune disease means no single therapy available at the moment (small-molecule or antibody alike) which has satisfied this niche. You can read more on different biological and non-biological treatments for Rheumatoid by the Arthritis Foundation.
As lead author on the study, Roy Fleischmann from the University of Texas Southwestern Medical Center, put it:
Despite the availability of a wide range of treatments, new agents are still needed to address unmet patient needs including failure to respond to therapy“
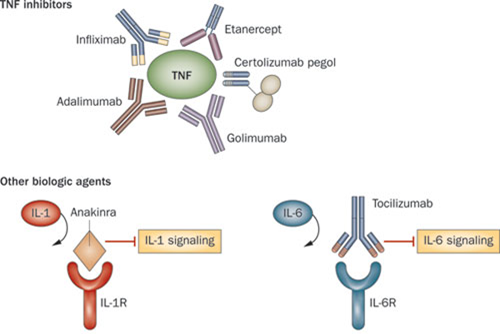
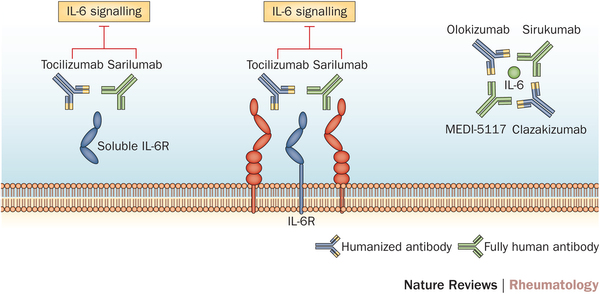
Sarilumab is a humanized monoclonal anti-body which acts as an interleukin-6 inhibitor (IL-6), therefore reducing the inflammatory response against tissues in Rheumatoid arthritis. Roche also has an IL-6 inhibitor, Tocilizumab, which is again prescribed for moderate to severe cases in conjunction with Methotrexate (the major anti-folic acid drug).
Both drugs are designed to treat Rheumatoid sufferers when other rheumatoid drugs (such as TNF-alpha inhibitors and IL-1 inhibitors) do not work well enough.
In the trial, 546 patients who were inadequate responders to TNF-alpha inhibitors took part, with promising results. However, treatment-emergent adverse events (TEAEs) in the drug reception included infection (ranging from 22 – 30% across the groups), although only 3 severe cases of infection under Sarilumab treatment were reported (as well as 2 cases in the placebo group).
Therefore, the most common reasons for discontinuation of the Sarilumab trial course was infection and neutropenia (depleted white cell count), which wasn’t exactly unexpected following the pre-clinical and mechanistic studies of the drug.
UPDATE (Original Publication 10/11/2015):
The FDA has handed down a rejection of sarilumab due to manufacturing problems, for which Sanofi previously received a citation. This comes as the latest blow in the pharma’s rough year, in which it lost out on the Medivation acquisition, suffered technical difficulties in its diabetes effort, and began to anticipate that its potential blockbuster, Praluent, could fast become a commercial failure.