CAR-T is a groundbreaking personalized cancer therapy with tremendous potential to drive the patient’s own immune cells to specifically target and kill cancer cells. Because the development of this therapy is still in the early phase, new tools are needed to provide a quick potency assay to assess the cytotoxicity of the therapeutic T-cells prior to infusion into the patient and to monitor their long-term cancer killing potential.
CAR-T are engineered T-cells which are genetically modified ex vivo to express proteins on their surface. These specifically recognize cancer cells (see our review for more) and kill them via a cytolysis process once they are infused back into the patient’s body. This modification of T-cells has initiated development of a whole new range of treatments against different types of cancer.
However, the major obstacles that need to be addressed in the development of this promising anti-cancer therapeutics, is ensuring that these cells will achieve their goal by attacking the right target and will persist inside human’s body. In addition, it is crucial to develop a protocol and process for the batch production of the engineered T-cells and to make sure that they meet the highest standard and quality.

How can we know that a CAR-T cell correctly and precisely targets the tumor?
Chromium-release assays (51Cr) are the ‘Gold-Standard’ for researchers within the field who want to measure immune cell-mediated cytotoxicity in vitro. In these assays, the target (cancer cell) are radioactively labeled and then mixed with effector cells (T-cells isolated from the immune system). The release of the radioactive isotope, which correlates with effector cell-mediated cytolysis of the targeted cell, is then measured typically after 4 hours.
However, this assay has several disadvantages, such as being time-consuming and expensive (cost of radioactive materials and disposal of radioactive waste), having high background noise after 4 hours measurement, and the fact that only a single data point or a ‘snapshot’ of the cells cytotoxic effect is obtained.
Some labs have instead developed flow cytometry methods and luciferase-based cytotoxicity assays, but these also have similar limitations. In the event that some tumor cells could withstand the initial assault by the T-cells and subsequently recover, it would be missed by endpoint assays such as 51Cr.
Looking beyond end-point analysis: Capturing T cell mediated cytotoxicity in action and “Going to the Movies”.
The xCELLigence real-time Cell Analyzer (RTCA, ACEA Biosciences, Inc.) allows the cell-mediated cytotoxicity window to expand beyond a single time point to a real-time continuous monitoring of how the CAR-T cells are working in vitro and when they will efficiently kill the target tumor cells.
The volume of information which can be captured by xCELLigence is similar to getting a feeling about a situation from a photograph versus a video…
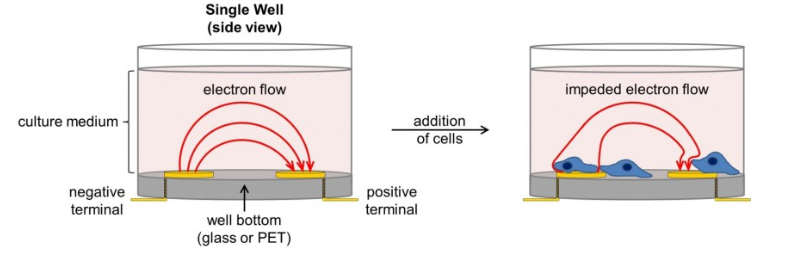
Further advantages with this tool are that it requires a minimal number of cells, allows you to work with a small Effector: Target ratio, saves time, runs totally dye-free under physiological conditions and provides a kinetic readout of combination therapies (check-point inhibitors & CAR-T).
How has xCELLigence improved screening and evaluation of Cancer Treatments?
Two great publications can be quoted to answer this question.
Published recently in Neuro-Oncology, Everson et al. combined the selective in vivo induction by Decitabine (DAC) of New York–esophageal squamous cell carcinoma (NY-ESO-1) antigen (an immunogenic tumor rejection antigen) in glioblastomas with the adoptive transfer of T-cells retrovirally transduced to express the NY-ESO-1 T-cell receptor.
Prior to in vivo studies, by using the xCELLigence platform for a read-out over 30 hours, they showed that epigenetic upregulation of NY-ESO-1 by DAC sensitize 2 glioblastoma cell lines to NY-ESO-1–T cells killing, at the physiological ratio of 1:1!
So, the real-time monitoring of the cytotoxicity on that specific glioblastoma target in vitro, has sped up the process to switch to a novel systemic treatment strategy for malignant gliomas.

The paper from Davenport et al. addresses the question of the respective role of the CAR versus that of the endogenous TCR information of the synapse and the subsequent activation of T-cells.
By monitoring over 50 hours, the cytotoxicity of their CAR-T cells presenting both receptors, they showed that both were equally effective in killing tumor cells in the short-term but a significant difference was observed between the CAR and the ‘real’ TCR between 20 and 50 hours of culture!
In fact, their CAR.OT-I cells down-regulated CARs to a higher degree than TCRs in response to cognate antigen in the long-term; explaining the loss of cytotoxic function in the long-term assays.
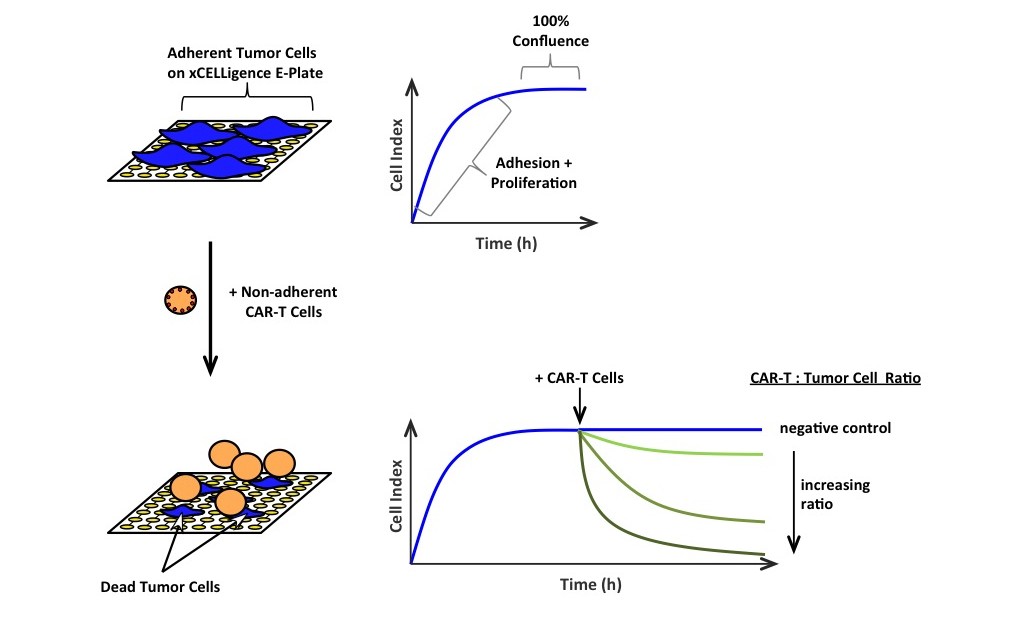
Long-term monitoring with xCELLigence allowed them to understand the loss of functionality of their ‘serial-killers‘ in 20-50 hours assays. The authors provide important insights into CAR-T – tumor cells interactions, kinetics of activation and tumor cell killing.
Conventional tests simply cannot capture this information, which is why assays like the xCELLigence Real-time Cell Analyzer (RTCA) can serve an important tool for Imuno-therapy assays…
As we mentioned, another great application of xCELLigence is for quality control of the CAR-T constructs. Indeed, it helps researchers to assess the degree of killing efficiency of each different batch produced and how good T-cells are achieving their goal.
So, in the world of CAR-T research (heralded a potential ‘Cure for Cancer’) the benefits of using real-time versus traditional assays are unparalleled. It’s a bit like comparing a snapshot with an IMAX film!






