CAR-T therapy has been hailed as a cure for cancer, but what really is this ‘miraculous technology’ and what can we actually expect from it?
The field of immuno-oncology is booming with billions of euros in investment. The ability to rewire our own immune system to fight cancer has certainly created huge expectations. After the success of the first checkpoint inhibitor drugs on the market, many are turning their attention to CAR-T cell therapy.
There are five CAR-T therapies already on the market. The field is now booming, with over 500 CAR-T clinical trials running worldwide. But is this therapy really a cure for cancer, as many seem to believe? Can the technology meet such high expectations? Are side effects a concern? Is it worth the huge price tag?
To answer the most burning questions, I talked with some of the leaders in this field to draw an overview of the current state of CAR-T technology.
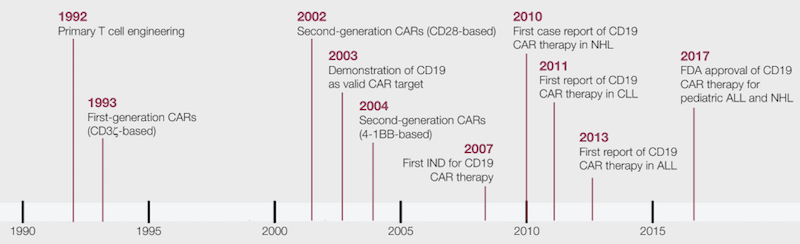
How does this ‘miracle cure’ work?
“CAR therapy is at the same time cell therapy, gene therapy, and immunotherapy. It represents a radical departure from all forms of medicine in existence until now,” Michel Sadelain, co-founder of Juno Therapeutics, told The Scientist.
A CAR-T therapy consists of the infusion of engineered T cells that carry a chimeric antigen receptor (CAR) on the cell membrane. The external domain of this receptor is designed to recognize a specific molecule on a tumor cell. When this happens, the internal signaling domain of the molecule is activated, stimulating the T cell to attack the cancer cell.
There are several generations of CARs, which carry additional internal domains that can further enhance the immune response against the programmed target.
The most common procedure for CAR-T cell therapy starts with the extraction of T cells from the patient being treated, a process called leukapheresis. The T cells are then genetically modified to express a CAR molecule and expanded. Finally, they are injected back into the patient, ready to fight the tumor.
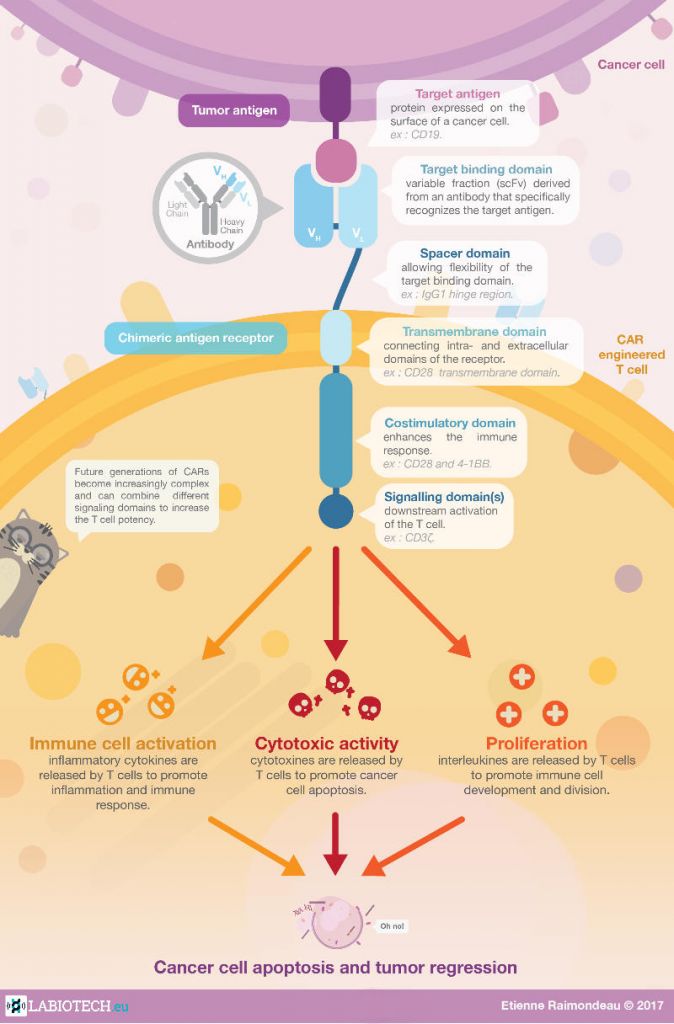
How have CAR-T cells changed cancer treatment?
Immunotherapies based on checkpoint inhibitors have been quite successful in certain groups of cancer patients. Checkpoint inhibitors block a mechanism that tumor cells use to hide from immune cells. Immunotherapies based on CAR-T cells go one step further, engineering the T cells themselves to enhance the natural immune response against a specific tumor antigen.
CAR-T clinical trials have shown huge remission rates, of up to 93%, in severe forms of blood cancer. This is particularly impressive considering most CAR-T clinical trials recruit cancer patients that have not responded to many if not all other available treatments. These results have fed the expectations of patients and investors alike, but it’s important to remember that the therapy can also have flaws.
André Choulika, CEO of French CAR-T developer Cellectis, put it bluntly: “I’m just trying to be realistic, CAR-T is not the miracle cure for cancer.”
Indeed, CAR-T cells have in fact been linked to severe and even lethal side effects, such as neurotoxicity and cytokine release syndrome. Over the years, several companies have reported deaths in late-stage clinical trials with CAR-T therapies. This has made many realize that the technology might not be as perfect as originally expected.
Many of these deaths were reported in trials testing CAR-T therapies against the CD19 antigen found in immune B cells — the most studied target in the CAR-T field. Four of the five CAR-T therapies currently on the market target CD19 to treat several forms of cancers affecting B cells, such as lymphoma and leukemia.
“The initial furor and excitement of CAR-T have led to extensive and rapid clinical development in the CD19 target space,” explained David Gilham, Chief Scientific Officer at Belgian CAR-T company Celyad.
“Research is busy catching up at the moment, in particular concerning toxicity. The lack of good preclinical models hampers this work, but with clinical samples available, ongoing investigations are now closer to identifying the underlying mechanisms and further refining the approach.”
Who’s developing CAR-T?
Novartis was the first to launch a CAR-T therapy, called Kymriah, in 2017. This one-time treatment for B-cell acute lymphoblastic leukemia (ALL) has shown an 83% remission rate after three months in patients that do not respond to standard treatments.
Gilead was the second to bring a CAR-T therapy to the market. The big pharma acquired CAR-T developer Kite Pharma just weeks before the FDA approved Kite’s CAR-T cell therapy, Yescarta. The therapy induced remission in 72% of patients with a rare form of blood cancer called aggressive B-cell non-Hodgkin lymphoma.
However, toxic effects on the immune system, like cytokine release syndrome and nervous system disorders, remain a worry for both Kymriah and Yescarta.
A recent study on the toxicity of these CD19 CAR-T treatments found that Yescarta resulted in a higher proportion of immune system disorders than Kymriah, while fatal toxic events were similar for both therapies. According to the study, the death rate for both therapies was 5.4%. As a comparison, that of immune checkpoint therapy lies between 0.36% and 1.08%.
In February 2021, the FDA approved the third CAR-T cell therapy. Breyanzi was developed by Juno Therapeutics, a Bristol-Myers Squibb company, for certain types of non-Hodgkin lymphoma. While the complete remission rate after treatment with the therapy was 54%, it can also cause severe side effects, including cytokine release syndrome and neurologic toxicities.
A month later, the FDA gave the green light for the very first CAR-T cell therapy that does not target CD19. Developed by Bluebird Bio and Bristol-Myers Squibb, Abecma targets B-cell maturation antigen (BCMA) and is the first CAR-T cell therapy for multiple myeloma. However, the therapy is only used after four or more prior treatments have failed, which is not surprising with a remission rate of only 28%.
The fifth CAR-T therapy to be approved was Kite Pharma’s Tecartus in early October 2021. Developed for adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (B-ALL), the therapy revealed a remission rate of 52% after three months of treatment. Although very severe adverse effects were not reported, 92% of patients did suffer from some form of cytokine release syndrome and neurologic toxicities occurred in 87% of patients.
A number of companies are running clinical trials with CAR-T therapy, including Autolus Therapeutics, TC Biopharm, and Adaptimmune in the UK, Cellectis in France, Celyad in Belgium, and Bellicum Pharmaceuticals and Mustang Bio in the US.
Making CAR-T therapy safer
Despite severe side effects and several deaths in clinical trials, some argue that CAR-T therapy is worth the risk for patients who don’t respond to any other available treatments. But CAR-T developers are already developing next-generation CAR T-cells that are safer for patients.
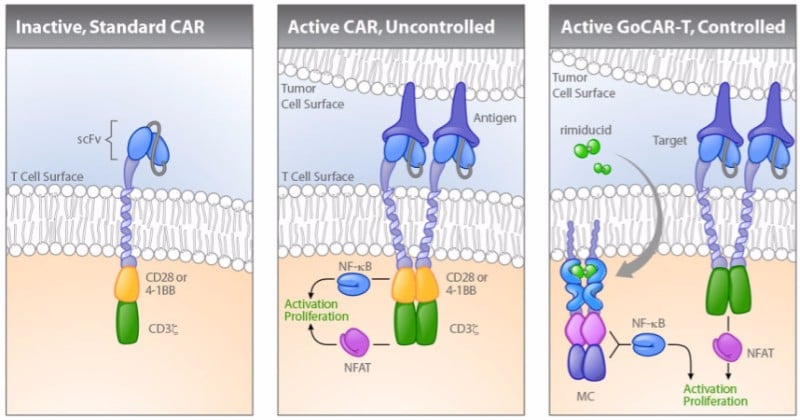
One of them is Cellectis. The company developed a CAR-T technology, now licensed to Servier and Allogene, that includes a switch control system that only activates the engineered T cells when the patient is given a drug. The therapy is in phase I trials, after saving two babies with aggressive forms of leukemia in compassionate cases.
Bellicum Pharmaceuticals in the US is developing a similar technology called GoCAR-T that requires the drug rimiducid to activate the CAR T-cells. Unlike other CAR-T cell therapies, Bellicum’s approach targets solid tumors. The company’s lead GoCAR-T candidate is currently in a phase I/II trial for patients with a specific type of pancreatic cancer.
Targeting solid tumors is one of the big challenges in the field of immuno-oncology. In these cancers, the amount of T cells that infiltrate naturally is low, and the tumor cells create an immunosuppressive environment that dampens the immune response. This makes solid tumors much more difficult to treat than liquid tumors.
Celyad is also targeting solid tumors. The company is developing a version of CAR-T that makes T cells carry the receptor of another type of immune cell called natural killer cells. “It’s disruptive because the receptor binds to eight different ligands that are expressed on above 80% of solid and hematological malignancies,” explained Christian Homsy, co-founder of Celyad. “We’ve started one of the largest and broadest trials in the sector, targeting seven indications.”
Another strategy is to combine CAR T-cells with other types of cancer immunotherapy. For example, by infusing them along with checkpoint inhibitors that block cancer’s defense mechanisms against T cells, the efficacy of CAR-T cells could be improved and the dose lowered. A drawback of this approach, however, could be making CAR-T therapy’s already prohibitive pricing even higher.
In fact, the pricing of the CAR-T therapies already on the market has sparked debate. In Germany, Novartis’ Kymriah is priced at €320,000 and Gilead’s Yescarta at €327,000. Experts estimate that, after factoring in the hospitalization and other medications required for the procedure, the total can exceed €1M per patient.
Off-the-shelf CAR T-cells
One big goal in the CAR-T field is the development of an allogeneic CAR-T therapy. That is, sourcing T cells from a healthy donor so that they are ready to go when the patient needs them, as opposed to engineering each patient’s T cells individually.
Cellectis has been the first to test two of these off-the-shelf CAR T-cell therapies in clinical trials, although there have been doubts over whether the technology can reach the same level of success as when using cells from the patient. One key challenge is to lower the risk of graft-versus-host disease, where the donor’s immune cells attack the patient’s body.
Celyad is also working on off-the-shelf CAR-T therapies. Two of its allogeneic candidates are currently in phase I and preliminary results seem promising so far, with no severe toxicity reported.
The off-the-shelf technology would save precious time for patients, who currently have to wait for about two weeks while their cells are shipped to the manufacturing facilities, engineered, and returned. But developing an off-the-shelf therapy is a “scientifically challenging avenue” according to Celyad’s co-founder Christian Homsy.
“I doubt that allogeneic CAR-T can be a real off-the-shelf therapy,” he told me. “There are still some significant scientific challenges with regards to immunology, as well as manufacturing, transportation, traceability, and banking solutions necessary to reach the scale needed for widespread patient treatment.”
In fact, the allogeneic CAR-T field suffered a huge scare in early October after Allogene discovered chromosomal abnormalities in the gene-edited, off-the-shelf CAR-T cells received by a lymphoma patient.
While the stakeholders are figuring out the severity of this finding, Allogene’s entire clinical pipeline was put on hold. This resulted in a massive hit to Allogene’s and Cellectis’ stocks, as Allogene was using Cellectis’ gene-editing technique.
As scientists try to understand how to overcome such technical challenges, the therapies also face challenges in terms of regulations. As a brand new type of therapy, CAR-T cells fall between several regulatory frameworks, which can widely vary from one country to another.
The challenges are many, and the technology is clearly not perfect yet, but CAR-T cells offer hope for patients that had none before. With the first therapies already on the market, the way is open for more and better alternatives to arrive in the next few years.
This article was originally published in January 2018 and has since been updated. rnrnImages via Shutterstock; Klebanoff et al. (2014) Nature Reviews Clinical Oncology 11, 685–686; Bellicum Pharmaceuticals





