Since the approval of the first gene therapy for blindness, there has been a wave of companies developing gene therapy treatments with the potential to cure different forms of genetic blindness.
Luxturna became the first gene therapy for inherited blindness to receive FDA approval back in 2017. About a year later, the therapy was approved in Europe. This was a significant milestone for innovation in the treatment of blindness but also for gene therapy, as Luxturna was also the first in vivo gene therapy — a treatment that is delivered directly into the patient’s cells rather than extracting and modifying the cells before reinjecting them.
Luxturna was developed by the US company Spark Therapeutics, which was acquired by Roche in 2019. The therapy is designed to treat patients with mutations on a gene called RPE65, which encodes a retinal protein necessary for the eye to respond to light. The treatment consists of injecting a healthy copy of the gene to restore the missing protein. A single injection in each eye has shown to be enough to improve vision for at least three years.
Although its exorbitant price has caused controversy, the success of Luxturna has set a precedent for many other gene therapies in development that are aimed at fixing the many other genetic mutations that cause blindness, with multiple approaches being put forward.
How gene therapy for blindness works
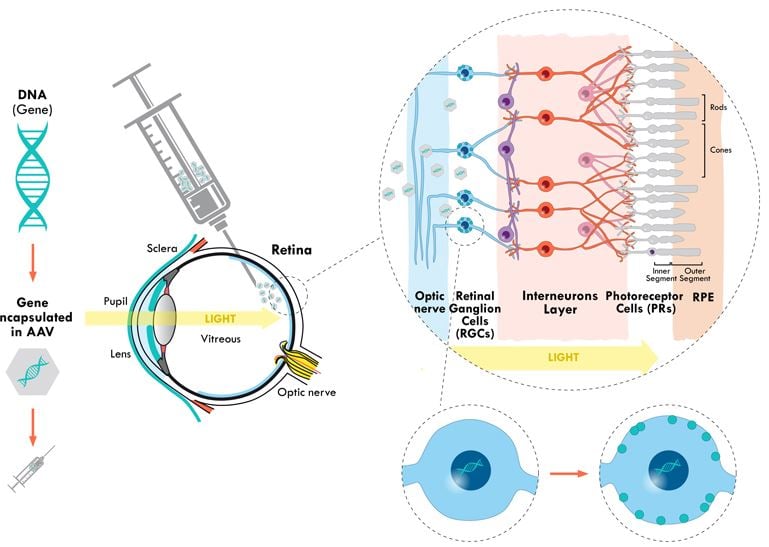
Gene therapy consists of providing the patient with a functional copy of a gene that is missing or mutated. In the case of Luxturna, a copy of the RPE65 gene is delivered to the patient’s eye using a viral vector. The virus is modified to eliminate its capacity for infection and introduce a functional gene that can be used by the patient’s cells to generate a functional protein.
Gene therapy is particularly suited to treat the eye. First of all, the eye is an immune-privileged area of the body where the immune system has restricted access. This prevents immune reactions against the viral vector used to deliver the treatment. In addition, the cells of the retina can keep this DNA functioning for longer than other cells in the body. This means that a single dose injected into each eye can be enough to restore sight in the long term.
“These cells don’t renew or mutate, and therefore should conserve DNA expression all their lifetime,” said Bernard Gilly, CEO of the French company GenSight. “A study on neurons in monkeys showed up to eight years of expression of the protein.”
Treating different forms of blindness
Following Luxturna, many biotech companies are advancing a variety of technologies to fix different mutations causing blindness. One of the most advanced is the Parisian GenSight, which is currently awaiting a decision from the EMA on a gene therapy for Leber hereditary optic neuropathy, a rare disease that causes irreversible vision loss.
GenSight suffered a setback back in 2018, when patients treated with the therapy in just one eye saw improvements in the eye that had been treated with placebo. Since then, the company has studied the rate of spontaneous recovery and deemed that it is too low to explain the improvement of untreated eyes, and thus supporting the concept that the gene therapy benefits both eyes. So far, the treatment has been shown to be safe and maintain its effects for at least five years.
Over in the US, Biogen is running a phase III trial of a gene therapy for choroideremia, a condition that impairs night vision. The pharma company got hold of this experimental treatment with the acquisition of Nightstar Therapeutics in 2019. Roche-owned Spark Therapeutics — Luxturna’s developer — is also targeting choroideremia, with a gene therapy being tested in phase I/II clinical trials.
Across the world, a number of companies are developing gene therapies targeting over 25 different types of sight-related conditions. Among the European biotech players, there are Syncona’s Gyroscope Therapeutics in London, and Horama and Eyevensys in Paris.
Among them, Eyevensys is developing an alternative method to traditional gene therapy that removes the use of viral vectors to deliver the gene therapy to the eye. The company’s technology consists of inserting the DNA directly into the ciliary muscle — a tiny muscle that surrounds the iris and adjusts the focus. This can be done through a minimally-invasive procedure that lasts less than two minutes, according to the company.
Is gene therapy for everybody?
Despite the great potential of gene therapy, one of its limitations is that each treatment can only fix a single gene. That significantly reduces the number of people that can benefit from each gene therapy when considering diseases like retinitis pigmentosa, which can be caused by mutations in 60 different genes.
Developing a gene therapy is expensive, making it unfeasible to develop one for each mutation, especially for extremely rare mutations. To overcome this challenge, some companies are designing new approaches to make a single therapy suitable for everyone.
In the case of GenSight, the company is developing a treatment that combines gene therapy with a wearable device to potentially treat all types of retinitis pigmentosa. The gene therapy is used to introduce a light-sensitive protein within neurons in the optic nerve. A pair of goggles then “helps redirect and concentrate the light toward the modified cells, which will then convert it into a signal for the brain,” said Gilly. A phase I/II trial is currently testing this technology.
Sounds great, but how much will it cost?
Gene therapy is unfortunately not cheap. In Germany, Luxturna costs a whopping €345,000. Per eye. That makes Luxturna one of the most expensive drugs in the world, along with other gene therapies such as Novartis’ Zolgensma or bluebird bio’s Zynteglo.
We can expect upcoming gene therapies to have similar price ranges, especially for those treating rarer forms of genetic blindness. These prohibitive figures might mean that not everyone will have access to curative treatments.
One of the solutions some companies are testing include spacing out the payment over time and conditioning the payment to the therapy working. For example, Spark Therapeutics has set up a payment scheme for Luxturna in the US that is based on the outcome of the gene therapy. The company will offer partial refunds depending on whether the therapy works in the short term (30-90 days) or the long term (30 months).
A brighter future in sight
In the coming years, we can expect more and more examples of gene therapy for blindness to enter the market and start providing curative solutions for many patients in need. One of the most anticipated technologies is CRISPR-Cas9, which has revolutionized the world by making gene editing simpler than ever.
With CRISPR it would be possible, in theory, to fix a mutation causing blindness directly in our retinal cells. Although the technology is still in the early stages and the first CRISPR treatments are directed at other conditions, US-based Editas Medicine is working with Allergan to develop a CRISPR therapy for blindness caused by Leber congenital amaurosis.
Still, the use of gene therapy will likely be limited to a small number of patients with specific mutations and rare diseases. It will likely take a long time before gene therapy can become a mainstream treatment that can really cure multiple causes of blindness. However, as time goes on, advances in technology will help expand the indications that can be treated with gene therapy.
This article was originally published in April 2018 and has since been updated. Cover image from Elena Resko. Body images via GenSight and Spark. Data visualization from Jon Smith.





