Known for curing US President Jimmy Carter’s cancer, Keytruda continues to benefit many other cancer patients. The inventors told us their story, from the drug’s early days to their current role as executives of the biotech unicorn Aduro.
Since 2014, the cancer drug Keytruda, also known as pembrolizumab, continues to be widely sought after due to its impressive effects. A form of antibody, Keytruda blocks the cancer cells’ ability to hide from the immune system. In melanoma patients treated with the drug, tumors are more likely to shrink compared to patients receiving chemotherapy and disease progression is reduced in many patients by 40% or more.
While Merck & Co has been responsible for Keytruda’s commercial success, the drug was originally developed by a team of Dutch scientists now at Aduro Biotech, which develops immunotherapies for cancer and is also using its technology to target autoimmune and infectious diseases. Keytruda is part of a group of drugs called checkpoint inhibitors that have shown impressive results in some cancer patients. While it was initially approved to treat melanoma by the FDA, it was approved for the treatment of any unresectable or metastatic solid tumor that had a specific genetic abnormality in 2017, the first time the FDA had approved a cancer drug purely on the basis of tumor genetics.
The three developers, Hans van Eenennaam, John Dulos and Andrea van Elsas, who respectively work at Aduro as Executive Vice President, Director and Chief Scientific Officer, told us about the drug’s history and about their experience of being at the top of one of the few biotech unicorns. Let’s take a look at Keytruda’s origins and its road to success!

How did Keytruda begin and where has it taken you?
Andrea van Elsas: We started the work in 2003 at a company called Organon, a Dutch pharmaceutical company with a tradition in women’s health and neurological diseases. In those days, immunology thought about how to develop antibody technology into a novel therapeutic agent. Keytruda was one of the avenues that we embarked upon, and it was eventually picked up by Merck & Co.
Hans van Eenennaam: We were one of four companies that broke through in immuno-oncology 125 years after the discovery that the immune system could be used to fight cancer. In 2011, we founded BioNovion in Oss, the Netherlands, to further develop the antibody technology from Organon; we improved and enhanced it to become the platform that is now one of the pillars of Aduro.
Could you elaborate on how your programs from BioNovion mesh with Aduro’s?
Andrea van Elsas: Aduro saw BioNovion’s programs as complementary to the company’s other platforms. Aduro saw the opportunity to do rational combinations of small and large molecules and whole cells: we believe these are essential to expanding the benefit of immunotherapy to more patients.
Aduro’s primary focus is in developing drugs that can activate the STING pathway, a moderator of innate immunity. We have a unique small molecule approach that is now progressing through Phase I trials in partnership with Novartis.
The core premise is that the lead drug candidate will activate inflammatory responses in the injected tumor and induce an immune response to target more distant tumors and lead to durable long-term immunity. Aduro believes its drug candidate can be combined with immune checkpoint inhibitors to induce immunity.
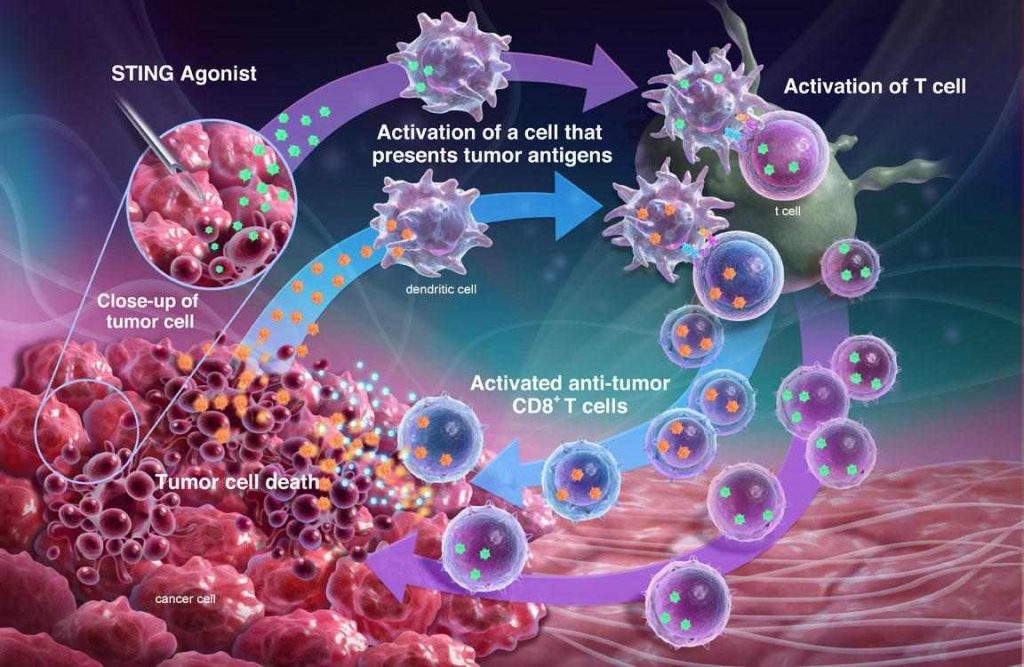
Hans van Eenennaam: Our lead antibody program, targeting the APRIL gene, a key driver in multiple myeloma and other tumors, is being explored in multiple myeloma and has the potential to address a variety of indications. A third program within Aduro’s clinical pipeline is based on listeria, a bacteria known by immunologists as a highly immunogenic vehicle to drive immune responses.
We have a personalized version of listeria in which neoantigens are sequenced out of a patient’s own tumor and then engineered into a personalized strain of listeria, which can then be used for therapeutic vaccination. This is now in Phase I trials. We believe this could be combined successfully with a checkpoint inhibitor drug in the future.
You don’t see small molecules as outdated then?
John Dulos: All of us made our careers in big pharma, and our view of the trends is that you should select an approach based on where biology is bringing you. So if you can only or preferably do it with a small molecule, that should be the driver. We are in a unique position as a biotech with three completely different technologies under one roof, so we can select the right technology for the challenge. All approaches equally feasible, it’s just a matter of finding the right targets.
As some of the founders of immuno-oncology, can you comment on the hype?
Hans van Eenennaam: It reflects the initial success with immunotherapy, which was achieved mostly with antibodies. By blocking the brakes on the immune response, we can accelerate it and provide long-term clinical benefit. CTLA4, an immune checkpoint that helps control immune responses, was the first checkpoint inhibitor to show the potential for immunotherapy. Nowadays, you’ll see that a lot is being done to educate physicians on safety issues, but we believe that the benefits of our programs outweigh potential side effects.
Andrea van Elsas: We have observed a manageable safety profile with our attenuated listeria strains to-date; it’s too early to say with the other programs. But of course, we will be very mindful of monitoring these as it is always an important consideration.
John Dulos: Everyone has been very excited about the breakthroughs in immunotherapy. We are proud to be part of the group that opened up the field and started the buzz.

Why did you move to the US?
Andrea van Elsas: That’s not entirely correct — the core discovery and research capabilities supporting our original platform are still driven from Oss in the Netherlands. In November 2015, the founders and shareholders of BioNovion decided to merge with Aduro, located in Berkeley. The STING and listeria-based cancer vaccine programs are driven there, and we have integrated development activities for clinical stage antibody programs across both sites.
Hans van Eenennaam: Moreover, we have nearly doubled the headcount of our research team in the Netherlands, reflecting a further investment in our people, structure and antibody programs. This underscores the commitment that we have to invest in the European franchise, and our collaboration with Merck is great news for European biotech and research in immuno-oncology: it has placed its biotech squarely in the limelight.
Thanks to Hans, John and Andrea for taking the time to tell us about their history!
The interview was originally published in January 2017 authored by Evelyn Warner. It has since been updated to reflect the latest developments in Keytruda and Aduro’s story.
Images via Nir Levy, Andrei Tudoran, Eric Valenne geostory / Shutterstock; Aduro





