A new transparency study has identified Sanofi, Novartis and GSK as the companies that keep the most clinical trial results unreported.
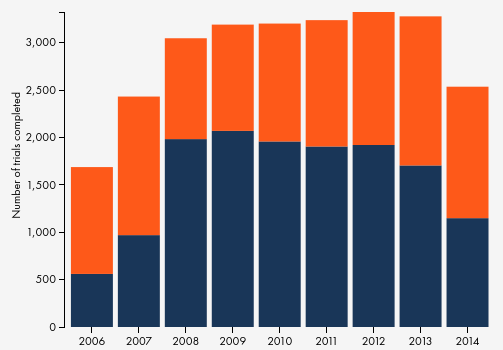
TrialsTracker is a tool developed at the University of Oxford that automatically rates transparency on clinical trials by checking if there are results available in clinicaltrials.gov and PubMed. The results: globally, 45% of data on completed clinical trials by major institutions, i.e. those with more than 30 trials, is missing.
The new tool has pointed at Sanofi as the worst offender, with 65% of its trial results from 2006 to 2014 kept secret. Novartis and GSK are the other pharma companies listed in the top 5 of secretive institutions, with 38% and 22.6% of data missing respectively.
On the other hand, US-based Shire is setting an example with 100% of its trial results publicly published. Bristol-Myers Squibb, Eli Lilly, Roche‘s Genentech and Johnson & Johnson are also among the most transparent companies.
The development of the algorithm was backed by AllTrials, an international initiative to promote the publication of results of all clinical trials. The transparency data published online is intended to raise awareness and pressure pharma companies into publishing results in order to improve their scores.
Why is transparency so important? If results are not available, doctors and patients can’t make informed decisions taking into account all the benefits and risks of a treatment. The data is also necessary for regulators and ethics committees to see the full picture before taking action.
The results from TrialsTracker highlight that there’s a huge bias in the information available from clinical trials. According to its website, negative results are twice as likely to not be reported, even though these are actually vital to avoid wasting money in what doesn’t work. If the initiative succeeds, researchers will have it easier to design trials that use resources efficiently and save patients time and pain.
Featured image by Tashatuvango/shutterstock.com
Figure from TrialsTracker





