From everyday immunostaining to billion-euro therapies, antibodies have become essential in Biotech. Selecting the right antibodies that are as close to human as possible minimizes risks and can be the key to success, especially in medical applications.
What goes into making the best therapeutic antibodies? To answer this question, we sat down with BIOTEM, an experienced provider of custom antibody services that develops fully humanized antibodies via its Ultimate Humanization® Platform. The company has developed a new technology to deliver the 4th generation of optimal therapeutic antibodies, and its answer is short: the best antibodies have low immunogenicity, high affinity, and low cross-reactivity.
It may sound simple, but many small biotechs don’t have the resources to develop high-quality antibodies with these characteristics. In particular, the development of lead-drug antibodies requires the optimization of toxicity and therapeutic effects to improve the results of preclinical and clinical studies.
But what is a 4th generation therapeutic antibody?
First of all, a bit of history. The first attempts used antibodies from animal origins that caused rejection, but at the end of the 20th century, antibodies started to be engineered. In the 1st generation of recombinant or chimeric antibodies, the constant sequences were substituted by their human counterpart. To improve upon this, in the 2nd generation the process was extended to variable sequences to create humanized antibodies. These grew into a 3rd generation comprised of human antibodies created in vitro with a library of naive, immature antibodies, or using transgenic mice.
It seemed like we had already reached the top. However, some 3rd-gen antibodies have been genetically engineered so much that it’s debatable whether they really are fully human; perhaps as a consequence, therapies from this generation present compatibility problems. 4th generation antibodies could address these complications by humanizing non-human primate antibodies.
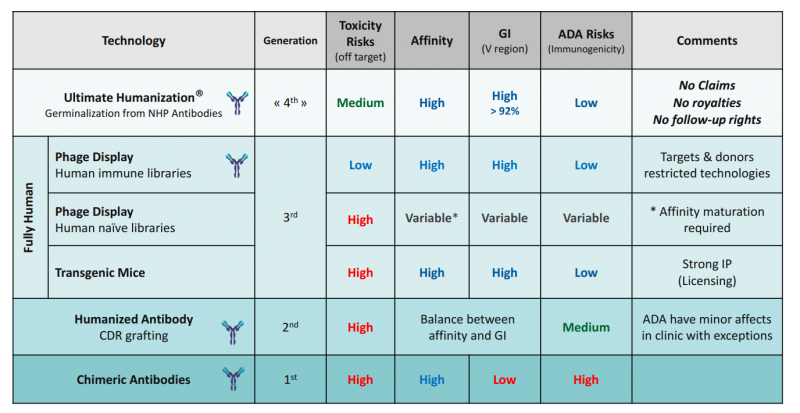
So how are optimal 4th-gen antibodies developed?
1. Low immunogenicity
A foreign antibody can induce an immune response against it, which inhibits its activity and generates adverse effects. Here comes into play the Germinality Index (GI), which measures the identity of an antibody with its most homologous human germinal sequences. Generally speaking, the higher the GI, the lower the risk of immunogenicity.
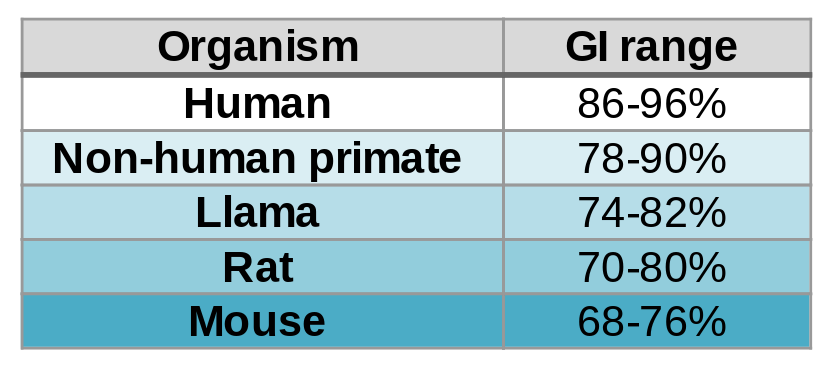
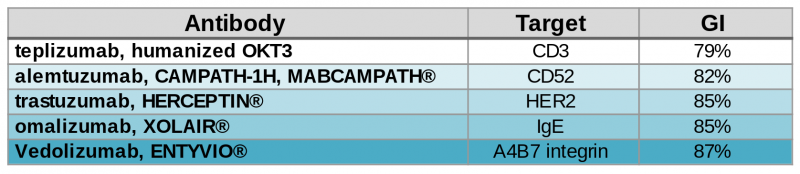
Contrary to what might be expected, fully human antibodies are not free from immunogenicity problems. In fact, most human IgGs themselves have a GI in the 88%-95% range due to somatic mutations.
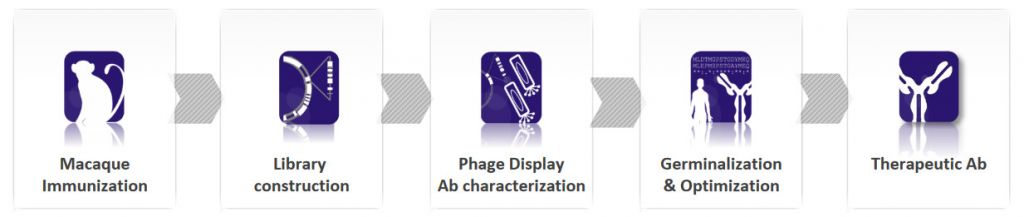
Now, BIOTEM has developed a way to bring the GI up to 99%. “How?” you may ask. First of all, as macaques are close to humans, they naturally produce antibodies with high GI. In addition, BIOTEM employs extended germinalization to mutate key FR and CDR regions and increase the GI without modifying the affinity.
2. High affinity
The affinity of an antibody to its target describes how strongly it binds to its antigen. In therapeutic applications, high affinity is important to ensure the antibody will detect and bind a higher percentage of target molecules, therefore inducing a stronger response with a lower dose.
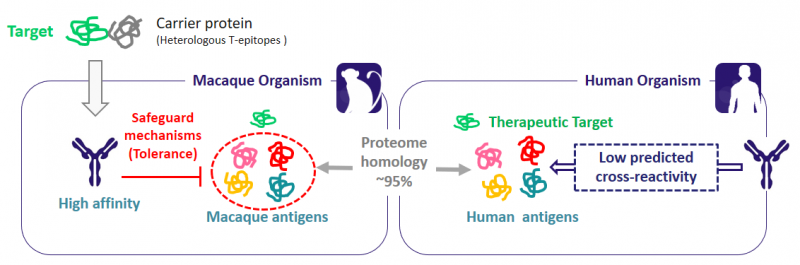
BIOTEM obtains the antibodies from actively immunized macaques, which allows the isolation of antibodies with higher affinity than those from a naive library. Furthermore, highly conserved target molecules can be conjugated with a carrier protein with heterologous T-epitopes to ensure a strong immune response. With this technology, BIOTEM can create high-affinity antibodies against any target: virus, bacteria, human proteins… you name it!
3. Low cross-reactivity
Therapeutic antibodies may bind to off-target entities, interfering with other biological processes and inducing undesired side-effects. These interactions are known as cross-reactivity. None of the classical antibody generations address this critical issue at an early stage of development, so herein lies an obvious need for improvement.
Luckily enough, here comes the Ultimate Humanization® Platform. Based on the universal tolerance mechanism, auto-reactive antibodies generated in macaques are naturally eliminated. Since macaques share a 95% protein sequence identity with humans, cross-reactivity with human proteins is extremely limited. This minimizes the toxicity risks compared to that of human antibodies derived from in vitro libraries or transgenic mice.
Finally, the antibody sequence is optimized to improve the physicochemical properties of the antibody. The aim is to improve manufacturability and stability and remove any residual immunogenicity. Codon optimization is also used to improve expression levels in the desired system.
All right, but why antibodies?
Nature Reviews Immunology has labeled therapeutic antibodies one of the greatest achievements in medical research. They’ve been used to tackle the huge challenges of arthritis, psoriasis, Crohn’s disease, leukemia, lupus and cancer so far.
As a result, antibody treatments have reached the top positions in the best-selling biological drugs, with Humira leading the list with a solid €13B in revenues last year. But competition is fierce and investors are keen to put millions in for the best antibody therapy candidates.
With such a complete service, BIOTEM helps Biotech companies minimize risks and provide them with flexibility for the development of successful antibody therapies. Check out their page to learn more about their technology and custom services.
Learn more about the Ultimate Humanization® Platform and drop BIOTEM a line!
Featured image: edited from Sebastian Kaulitzki/shutterstock.com
Figures 1-5 courtesy of BIOTEM