News and Trends 2 Aug 2022
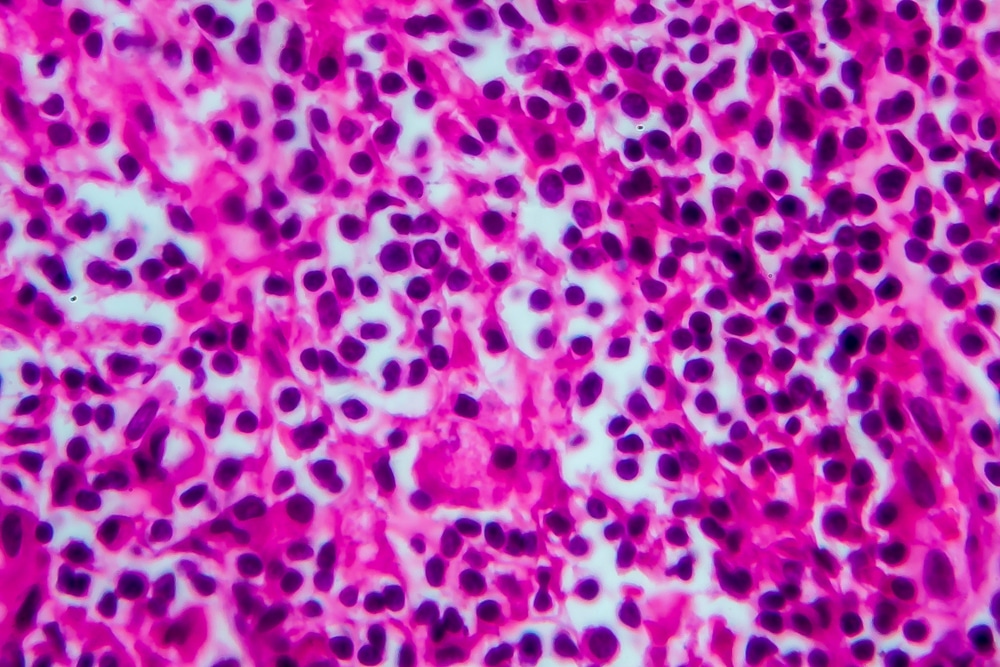
Cellectis gets green light for non-Hodgkin lymphoma trial
French-headquartered gene-editing biotech company Cellectis says the U.S. Food and Drug Administration (FDA) has cleared Cellectis’ Investigational New Drug (IND) application to initiate a phase 1/2a clinical trial of UCART20x22 for patients with relapsed or refractory non-Hodgkin lymphoma (r/r NHL). The company plans to begin enrolling patients in the NatHaLi-01 study in the second half […]