News and Trends 23 Jun 2022
Krystal Biotech looks for FDA approval for dystrophic epidermolysis bullosa treatment
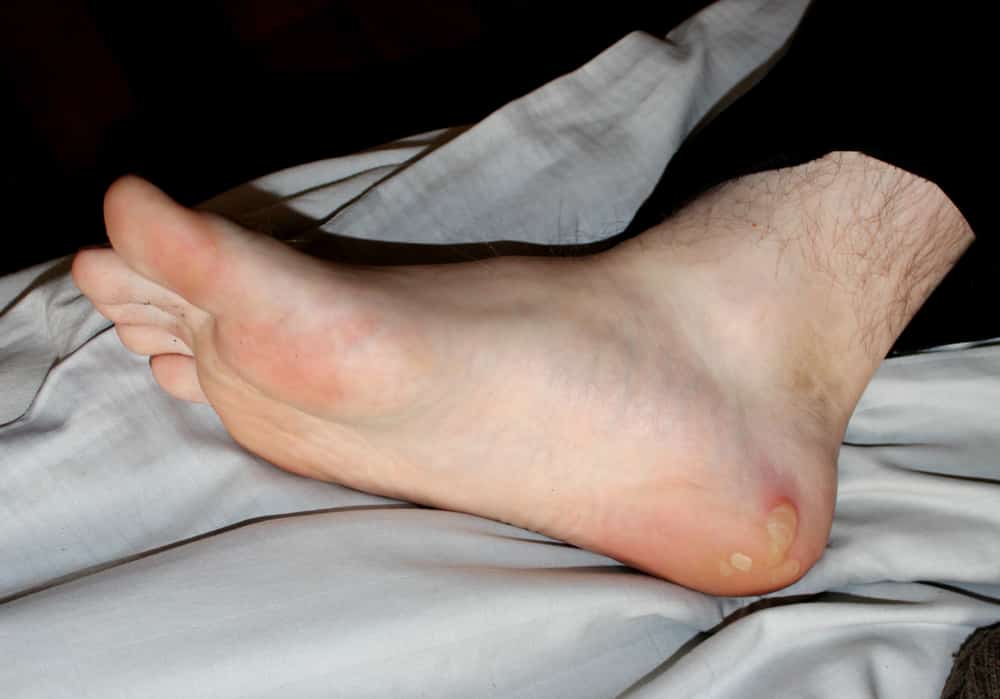
U.S. gene therapy company Krystal Biotech, Inc, has submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) seeking approval of B-VEC (beremagene geperpavec) for the treatment of patients with dystrophic epidermolysis bullosa (DEB). B-VEC is an investigational non-invasive, topical gene therapy designed to treat DEB at the molecular level by […]